Webinaire EMA le 20/01/2023 : Utilisation du portail CTIS (Clinical Trial Information System) • GIRCI Île-De-France

ANSM Agence nationale de sécurité du médicament et des produits de santé sur LinkedIn : Use of Clinical Trials Information System becomes mandatory for new…

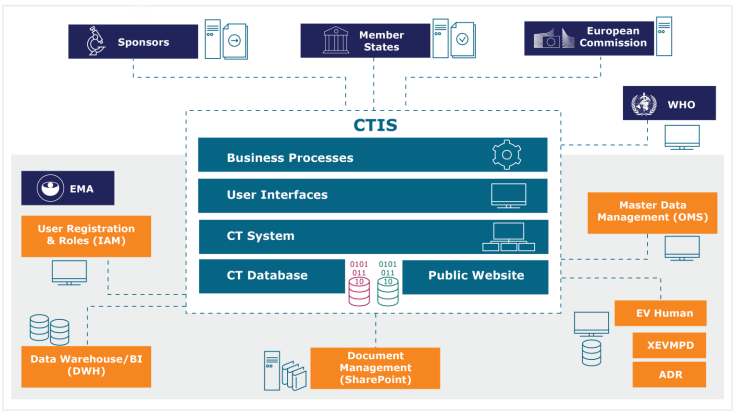
Managing the New EU Clinical Trials Regulation 536/2014 – Guidance for Navigating the Clinical Trial Information System (CTIS)